Comprehensive Biologics Characterization
Our comprehensive characterization toolkits support the evaluation of key quality attributes in therapeutic biologics, ensuring quality, safety, and efficacy.
Biologics Intact Mass Analysis
Sample preparation options
Intact mass measurement under reduced and non-reduced conditions
Deglycosylated and native (non-deglycosylated) intact mass analysis
Enzymatic digestion of monoclonal antibodies (mAbs) into subunits using IdeS or IgdE
Data acquisition, process and report
An ultra-high-pressure liquid chromatograph coupled with a high-resolution mass spectrometer
Automated data processing followed by expert scientific review to ensure high data quality
Accelerated turnaround times to meet client expectations
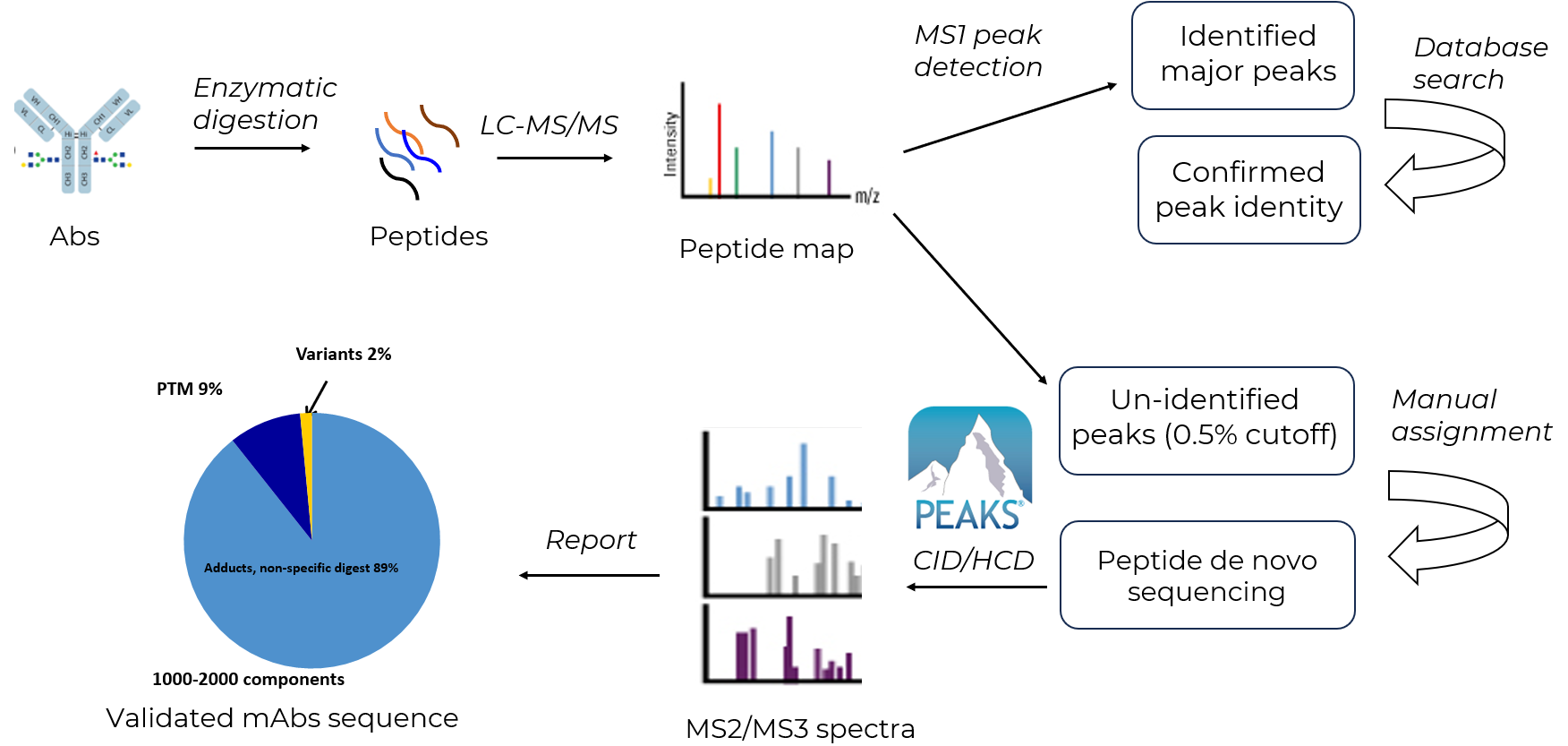
Peptide Mapping Strategy
Analysis of Disulfide Linkages and Scrambling
Profile comparison approach: Peaks present in the non-reduced sample but missing from the reduced counterpart are interpreted as disulfide-linked peptides.